Blood-brain barrier and ADHD
The blood-brain barrier is a group of mechanisms that separates the blood from the cerebrospinal fluid. The blood-brain barrier protects blood vessels in the brain very tightly against the uncontrolled passage of substances into the brain. The exchange is controlled by transporters and vesicles.
The mechanisms of the blood-brain barrier are located in or around the endothelial cells. Some mechanisms induce (tight junctions, transporters, metabolic enzymes) and others inhibit (transcytosis, LAM) the exchange of substances1
The same barrier on the spinal cord is called the blood-spinal cord barrier.
BBB injury correlates with the accumulation of numerous vascular and neurotoxic molecules in the brain parenchyma, reduced cerebral blood flow and hypoxia.2
A basic introduction to the blood-brain barrier in German can be found at Psysiologie.cc3, in English at Daneman, Prat.1
- 1. Components of the blood-brain barrier
- 1. Tight junctions and their proteins
- 2. Transporter through the blood-brain barrier
- 3. Transcytosis (caveolin-based vesicle transport)
- 4. Leukocyte adhesion molecules (LAM)
- 5. Own immune reaction of the blood-brain barrier
- 6. Regulation of the blood-brain barrier
- 7. Functional disorders of the blood-brain barrier
- 8. Bypassing the blood-brain barrier
- 8. Blood-brain barrier in various disorders
1. Components of the blood-brain barrier
The most important elements of the blood-brain barrier (BBB) are:
- Basement membrane
- Endothelial cells
- lie on the inside of the basal membrane of the capillary blood vessels of the brain
- are closely connected to each other by tight junctions
- enclose the lumen (cavity through which the blood flows)
- Pericytes
- Pericytes enclose the basement membrane of the capillaries from the outside
- Are surrounded by the Virchow-Robin space
- Interruptions in pericyte coverage increase non-specific transcytosis in the cells and the expression of leukocyte adhesion molecules. Contribution to neurological diseases open.
- Astrocyte processes
- Support the capillaries
- Disruption of astrocyte endfeet at the NVU decreases glymphatic clearance. Possible contribution to pathological accumulation of proteins, including Aβ2
- Microglia
The sum of neurons, endothelial cells and astrocytes in the BBB is called the neurovascular unit (NVU).
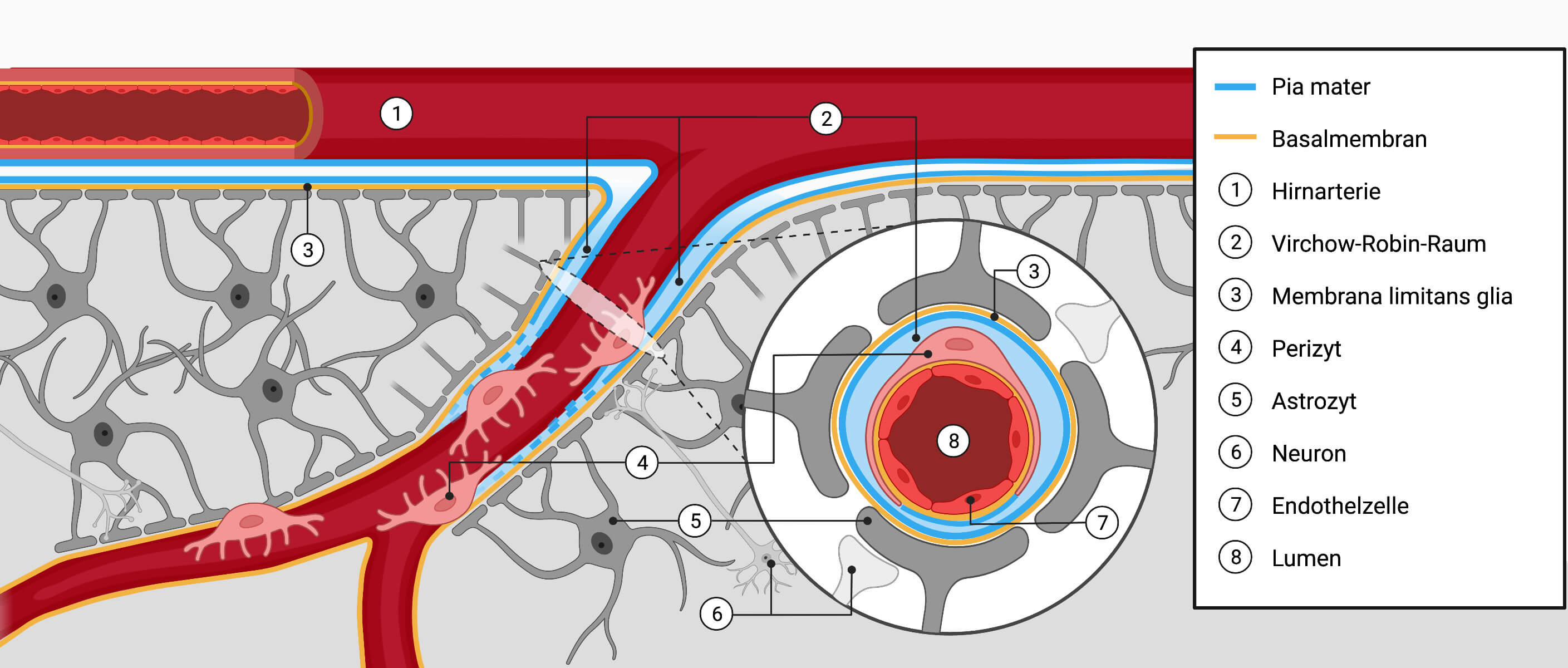
Image author: Janica Nolte, DocCheck, created with BioRender.com; licensed under CC BY-NC-SA 3.0source:DocCheck Flexikon: Schematic representation of the brain tissue layers and vessels
1. Tight junctions and their proteins
Tight junctions (lat.: zonula occludens) are mechanisms that anchor brain endothelial cells to the cytoskeleton via tight junction proteins, which stabilizes the cell structure. As a result, the endothelial cells form a tight barrier.
This prevents substances from bypassing the endothelial cells and entering the cerebrospinal fluid.
Tight junctions minimize diffusion from the blood vessel past the endothelial cells. This diffusion is a measure of the integrity of the BBB and can be measured with the transendothelial electrical resistance (TEER). TEER in the BBB is 1,500 to 2,000 Ω/cm2 and is therefore 50 to 500 times higher than in peripheral blood vessels (3 to 30 Ω/cm2).
(1,500-2,000 compared to 3-30 Ω/cm2).4
In addition to tight junctions, other cell contacts are adhesion contacts and gap junctions. The latter are communication connections that electrically couple cells together.
Tight junctions form looser connections in the intestine and tighter connections in the brain.
However, they are not only found in epithelia and endothelia, but also in myelinated cells. Axons are enveloped by a loose spiral of oligodendrocytes and Schwann cells. This multilamellar structure insulates the axon electrically and enables saltatory stimulus transmission. The tight junctions between glia and axons and within the myelin sheaths contain proteins similar to those found in the tight junctions of epithelia and endothelia.5
Tight junctions consist of transmembrane proteins such as5
- Claudine
- Occludine
- Tetraspanin
- JAM (junctional adhesion molecule)
- CAR
- Protein 0 (P0)
- OSP/claudin 11
- PMP22/gas-3
- OAP-1/TSPAN-3
- ZO-1
- ZO-2
- ZO-3
- Pals1
- MAGI-1/BAP-1
- MAGI-2
- MAGI-3
- PAR proteins
- MUPP1
- AF-6/Afadin
- PATJ
- Cingulin
- Symplekin
- 7H6 antigen/barmoti
- Rab proteins
- Pilt
- JEAP
- huASH1
- Heterotrimeric G proteins
- ICAM, intercellular adhesion molecule2
2. Transporter through the blood-brain barrier
Transport proteins are involved in the absorption into and excretion from the organism as well as the transfer of drugs from the blood into the tissue.
2.1. Efflux transporter
Efflux transporters utilize the hydrolysis of ATP to transport their substrates across the concentration gradient. Many of these transporters are localized at the luminal surface and transport a wide range of substrates into the blood:1
- P-glycoprotein (MDR1 gene): controls the transfer of drugs into the brain
- MDR1 gene variants influence its efficacy. Reduced MDR1 function weakens the blood-brain barrier, drugs can increasingly enter the brain, which can increase their effect even though the blood plasma level is unchanged.6
- BCRP, breast cancer resistance protein
- MRP, multidrug resistance-associated protein
2.2. Nutrient transporter
Nutrient transporters facilitate the movement of certain nutrients along their concentration gradient:1
- Solute carrier transporter
- GLUT1 (glucose transporter 1, SLC2a1): Glucose
- SLC16a1: lactate, pyruvate
- SLC7a1: cationic amino acids
- LAT1 (Large Neutral Amino Acid Transporter 1, SLC7a5): neutral amino acids (phenylalanine, tyrosine, tryptophan), L-DOPA (non-proteinogenic α-amino acid), branched-chain amino acids (BCAAs)7
- forms a heterodimer with the glycoprotein CD98, which is encoded by the SLC3A2 gene
- BCAAs are transported into the developing brain at a much higher rate than in adulthood
- SLC7A5 appears to function as an antiporter and histidine appears to be the main counter-amino acid
- SLC7A5 does not facilitate the transport of neurotransmitters
- Receptor-mediated transport systems
- Transferrin receptor: transferrin/iron
- Ager: Amyloid
- LRP1/LRP8
2.3. Competition for van use
Competition between different substances with regard to transport through the brain barrier can influence the effect of medication.
Example: Memantine, opiates (oxycodone, codeine), tramadol, cocaine and nicotine are transported through the blood-brain barrier by the same transporter family (organic cation transporter, OCT). Since OCT are subject to a saturation limit, this common transport mechanism could influence the memantine level in the brain and thus its effect.8
One person with ADHD told us that the administration of memantine helped her well against her ADHD symptoms.
In SHR, the most studied ADHD model animal, the expression of the lactate transporter monocarboxylate transporter 1 (MCT1) at the blood-brain barrier in the hippocampus is increased. The authors hypothesize that hyperactivity due to increased lactate production in skeletal muscles as a result of exercise could be a response to compensate for a reduced supply from astrocytes.9
3. Transcytosis (caveolin-based vesicle transport)
In the endothelial cells of the brain, the transcytosis rate is drastically lower than in peripheral tissues. Transcytosis is upregulated during injury or disease and then represents the greatest weakness of the blood-brain barrier.
Caveolin-1 is expressed by all oocytes and is upregulated in the blood-brain barrier after traumatic brain injury. Similarly, the plasmalemma vesicle-associated protein (PLVAP) is upregulated in a number of diseases in which there is a leak in the blood-brain barrier.1
4. Leukocyte adhesion molecules (LAM)
Leukocytes bind to the corresponding adhesion molecules of the endothelial cells via the adhesion molecules LFA-1, Mac-1 or CR4 present on their surface. The leukocytes then pass through the endothelium and migrate into the surrounding tissue (leukocyte migration).10
LAM are much rarer in brain endothelial cells than in endothelial cells in the body. However, neuroinflammatory diseases such as stroke and MS increase LAM in the CNS beyond the helpful level.
Depending on the disease, different subgroups of inflammatory cells infiltrate the brain1
- MS
- Lymphocytes
- T-cells
- B cells
- Neutrophils
- Macrophages
- Lymphocytes
- Stroke
- Neutrophils
- Macrophages
- but barely any lymphocytes
The transport of leukocytes, which is essential for fighting infections, is significantly increased in MS.
In order to specifically combat pathological inflammation without making patients more susceptible to infections, only the respective leukocyte adhesion molecules that facilitate the extravasation of the respective subset of immune cells should be inhibited:2
- NINJ1 (Ninjurin1): Monocytes
- ALCAM (Activated Leukocyte Cell Adhesion Molecule): CD4+ T cells, monocytes
- ALCAM is also required for tight junctions, ALCAM-KO mice show severe experimental autoimmune encephalomyelitis11
This shows that only inhibition and not elimination can be helpful.
- ALCAM is also required for tight junctions, ALCAM-KO mice show severe experimental autoimmune encephalomyelitis11
- JAML (Junctional Adhesion Molecule-Like, or AMICA1): Monocytes, CD8+ T cells
- MCAM (melanoma cell adhesion molecule); CD8, T helper cells 17
5. Own immune reaction of the blood-brain barrier
The BBB not only protects brain cells from peripheral influences, but also actively promotes neuroinflammation by controlling immune responses during autoimmune attacks on the CNS.
Brain endothelial cells can release various substances1
- proinflammatory chemokines
are necessary to guide lymphocytes and monocytes into the brain:- CCL2
- CCL5
- CXCL10
- proinflammatory cytokines
upregulate the expression of proinflammatory mediators and influence the expression of junctional proteins, thereby reducing the permeability of the BBB- IL-17
- IL-22
- Granulocyte-macrophage colony-stimulating factor (GM-CSF)
- Interferon (IFN)-γ
- TNF (tumor necrosis factor)
- intercellular adhesion molecules
mediate (at least in part) the adhesion process and transmigration of leukocytes and leukocyte subtypes to the CNS- ICAM-1
- ICAM-2
- vascular CAM (VCAM)-1
- activated leukocyte CAM (ALCAM)
- Melanoma CAM (MCAM)
- Ninjurin-1
6. Regulation of the blood-brain barrier
Signaling pathways and transcription factors can have different effects on the BBB1
6.1. Promotion of the BHS barrier
The BHS barrier is supported by:
- WNT (important)
- Binding to Frizzled/LRP5/6 activates β-catenin, which causes expression and targeting of the junctional proteins claudin-3 and p120 to the cell membrane
- Loss of the WNT co-receptor LRP5 reduces claudin-5 expression
- Hedgehog (important)
- controls the transcription and expression of junction proteins
- dampens inflammatory reactions on endothelial cells of the brain
- SOX-18
- controls the expression of claudin-5
- is activated by GLI1, which in turn is activated by Hedgehog ligands or WNT
- NR2F2 (Nuclear Receptor Subfamily 2 Group F Member 2)
- is induced by Hedgehog ligands or WNT
- promotes the expression of ANG-1 promotes
- induces the expression of junction proteins by TIE-2
- reduces the expression of ANG-2
- since ANG2 reduces the expression of junction proteins, NR2F2 also indirectly increases the expression of junction proteins
- activated by oxidative stress Antioxidant Response Elements (ARE)
- ARE stabilize the expression of ZO-1, occludin and claudin-5
- protects endothelial cells of the brain in the event of injury by suppressing the expression of inflammatory genes
- ERG
- NKX2-1
- SP3/YY1
6.2. Reduction of the BHS barrier
The BHS barrier is reduced by:
- NF-κB
- Snail
- Bacterial Induction of Snail1 Contributes to Disorder of the Blood-Brain Barrier12
- Snail is inhibited by β-catenin
- this reduces the stability of p120/VE-cadherin complexes and the expression of the tight junction molecules occludin and claudin-5
- FOXO1
- PKC
- eNOS
The blood-brain barrier is regulated, among other things, by perivascular cells that line blood vessels from the inside. These include pericytes and astrocytes.
Pericytes do not appear to be involved in the development of the BBB or its specific properties such as tight junctions or transporters, but inhibit factors associated with leaky peripheral vessels such as transcytosis or LAM1
Astrocytes influence the shape of endothelial cells, the formation of new blood vessels from existing blood vessels (angiogenesis) and the phenotype of the blood-brain barrier under physiological and pathological conditions.13
Astrocytes produce factors that influence endothelial function during development and in adulthood.
- VEGF, vascular endothelial growth factors 141
- VEGF family includes
- VEGF-A, VEGF-B, VEGF-C, VEGF-D, VEGF-E, PlGF
- bind to specialized tyrosine receptor kinases, VEGF receptor-1, VEGFR-2, neuropilins
- during development
- angiogenic: promote the formation, remodeling and survival of embryonic blood vessels
- neurotrophic factors
- in adulthood
- in inflammation reduce VEGF stability of the BBB
- 17β-estradiol reversed the increase in BBB permeability caused by VEGF1516
- VEGF modulated the migration of oligodendrocyte precursor cells (OPCs). OPCs support the integrity of the mature blood-brain barrier.14
- low VEGF correlated with ASA symptom severity, but only in women17
- VEGF family includes
- Hedgehog signal cascade1
Involved in embryonic morphogenesis, neuronal control and angiogenesis; induces tight junction proteins- Astrocytes secrete Sonic Hedgehog
- Endothelial cells express
- Hedgehog receptor Patched-1
- Smoothened (SMO), a signal transmitter
- SMO-KO mice showed increased permeability of the blood-brain barrier
- Transcription factors of the GLI family
- Angiopoietins. Angiopoietins are growth factors that control the sprouting of blood vessels into tissue, i.e. angiogenesis1
- ANG1: involved in
- BBB differentiation through promotion of angiogenesis
- time-dependent inhibition of endothelial permeability by increasing junctional protein expression
- Increase in BBB barrier function with simultaneous expression of VEGF
- ABG-2: involved in
- Reduction of the BBB in the event of injuries and illnesses
- ANG1: involved in
- ACE-1, angiotensin-converting enzyme-11
- converts angiotensin I into angiotensin II
- acts on angiotensin AT1 receptors expressed by the BBB elements
- Angiotensin I reduces BBB permeability and stabilizes the function of junctional proteins by promoting their recruitment into lipid rafts.
- Angiotensin II causes vasoconstriction and promotes tight junction formation
- Angiotensin-deficient mice show altered expression of occludin at the BBB
- TGF-β, transforming growth factor β1 is a cytokine that is involved in cell growth, differentiation, morphogenesis, apoptosis and immunomodulation
- is secreted by astrocytes and brain endothelial cells
- neuroprotective effect in the brain
- can induce MDR1 activity and reduce BBB permeability
- reduces leukocyte transmigration through the endothelium
- Retinoic acid1
- is secreted by astrocytes and radial glial cells
- Receptor: RA receptor β (RAR-β) is expressed in the developing vasculature
- RAR-β activation increases TEER, which correlates with increased expression of VE-cadherin, P-gp and ZO-1
- RA modulates the Hedgehog, Wnt and FGF signaling pathways
7. Functional disorders of the blood-brain barrier
BBB dysfunction is a central element of pathology in some diseases (e.g. MS, epilepsy and stroke).
In other disorders (e.g. Alzheimer’s and ASD), the significance of BBB dysfunction is being discussed.
Disorder of the blood-brain barrier causes
- Dysregulation of the ions
- Edema
- Neuroinflammation
This can lead to neuronal dysfunction, increased intracranial pressure and neuronal degeneration.2
The blood-brain barrier is not a uniform structure in the sense of a physical wall that would be destroyed in the event of a “BBB breakthrough”.
Rather, the BBB is the result of the interaction of a number of physiological properties. Changing just one property (transcytosis, transport) can significantly alter the neuronal environment.
Depending on which part of the BBB is impaired, this can lead to other disorders. Disorder pathways can be:2
- GLUT1 glucose transport
- LAT1 amino acid transport
- ASS7
- MCT8 thyroid hormone transport
- psychomotor retardation20
- VEGF-A, vascular endothelial growth factor
- MS21
- inflammatory cytokines
- Tumor necrosis factor α
- Interleukins 1 and 6
- Craniocerebral trauma22
- reactive oxygen species
- Matrix metalloproteinases
- Wnt signal transmission
The extravasation of leukocytes takes place via an active trafficking process2
There are many subgroups of immune cells with different roles in neuroinflammation:
- Parenchymal ECs upregulate leukocyte adhesion molecules and thus increase leukocyte trafficking
- P-selectin and E-selectin mediate the rolling of leukocytes along the endothelium
- ICAM1 and VCAM1 mediate firm adhesion
- The levels of ICAM1 and PECAM1 can influence whether the extravasation of leukocytes across the BBB is transcellular or paracellular.
- Proteins such as PLVAP help with transmigration through the ECs
- PLVAP are also upregulated in diseases
8. Bypassing the blood-brain barrier
The blood-brain barrier makes it very difficult for drugs to reach targets in the brain. One possible way to circumvent this is to administer drugs to the brain via the nose using nanoformulations. The drugs thus enter the nasal cavity and from there reach the brain via connections between the olfactory and trigeminal nerves and the nasal mucosa.23
Intranasal dopamine administration reduced hyperactivity and improved attention in Naples High Excitability rats, an ADHD model animal.24
8. Blood-brain barrier in various disorders
For vascular influences on ADHD, see Ahirwar et al, 202525
8.1. Blood-brain barrier and ADHD
In SHR, the blood-brain barrier is damaged by increased neuroinflammation and excessive autophagy. In the brain of SHR:26
- the number of Iba1-immunopositive microglia is increased
- TNF-α increased
- in the PFC and hippocampus
- Increased autophagy of cells
- MMP2 increased
- MPP9 increased
- Zonula occludens-1 (ZO-1, a tight junction protein) reduces
- Occludin (a tight junction protein) reduces
One study found evidence of reduced tryptophan uptake across the blood-brain barrier in boys with ADHD. Tyrosine uptake was unchanged.27
Increased zonulin levels and occludin levels as well as reduced claudin-5 levels correlate with an impaired blood-brain barrier.
8.1.1. Zonulin for ADHD
Zonulin controls the tight junctions. Elevated zonulin levels represent increased permeability of the intestinal wall and the blood-horn axis.28
Studies found
- increased zonulin levels in persons with ADHD, whereby the increased zonulin levels also correlated with hyperactivity,29 so that there could be a higher connection to ADHD-HI than to ADHD-I.
- elevated serum zonulin levels in children with ADHD30
- unchanged zonulin levels31
- slightly (not significantly) reduced zonulin serum values32
Elevated zonulin correlated with increased social difficulties in ADHD.33
In addition, reduced DEFA1 levels were found during inattention.32
Elevated zonulin levels could be compensated by an increase in Bifidobacterium.3435 Bifidobacterium was found to be increased in ADHD by some studies and decreased by others. More on this under Gut-brain axis and ADHD
More about Zonulin and its effects:
⇒ Increased intestinal permeability in ADHD
8.1.2. Claudine with ADHD
Reduced claudin-5 levels represent an increased permeability of the blood-brain barrier36
- reduced claudin-5 blood levels36
- elevated claudin-5 blood levels31
- unchanged claudin-5 blood levels (meta-analysis, k = 17)37
8.1.3. Occludine for ADHD
Studies found with ADHD
- elevated occludin blood levels (meta-analysis, k = 17)37
- slightly (not significantly) elevated occludin serum levels, which correlated with a subtest of the Conners’ continuous performance test32
Occludin levels correlated with ADHD symptoms.30
8.2. Blood-brain barrier and ASS
Impairments of the LAT1 amino acid transporter can lead to ASD due to a lack of branched-chain amino acids (BCAAs) in the brain.7
The Large Neutral Amino Acid Transporter 1 (LAT1) is encoded by the SLC7A5 gene, is located at the blood-brain barrier and forms a heterodimer with the glycoprotein CD98, which is encoded by the SLC3A2 gene.
BCAAs are transported into the developing brain at a much higher rate than in adulthood.7
8.3. Blood-brain barrier and MS
Multiple sclerosis is mediated by T cells, with CD4 T helper (Th) cells of the Th17 and Th1 phenotype being fundamentally involved.1
In addition, B cells play an important role in the immunopathogenesis of MS, as
- the antibodies produced in the brain are an essential feature of the disease (i.e. oligoclonal bands)
- b cell-targeted therapies strongly protect against lesion formation
During infiltration by immune cells and the formation of lesions, the blood-brain barrier is impaired, as evidenced by vascular leakage and changes in junctional proteins. Abnormalities in the expression of junctional proteins are associated with perivascular astrogliosis in MS. Such changes are detected at very early stages of lesion formation.1
In MS, reactive astrocytes express VEGF-A, which is capable of causing BBB breakdown and promoting immune cell invasion into the CNS by Disorder of claudin-5 and occludin expression.
VEGF-A binds to the VEGFR2 receptor on the brain endothelial cells of the BBB, which triggers the eNOS-dependent downregulation of the junctional proteins claudin-5 and occludin, leading to BBB collapse.
The upregulation of VEGF-A appears to be induced by IL-1 from microglia.
In MS lesions, the expression of AGT in astrocytes and occludin in brain endothelial cells is reduced compared to unaffected white matter. This correlates with reduced AGT expression in astrocytes stimulated in vitro with IFN-γ and TNF-α. AGT-deficient mice have impaired BBB function, which correlates with reduced and impaired expression of occludin.
There are types of lesions in which BBB permeability is initial, in others the impairment of the BBB only follows the lesions.38 In new lesions, the BBB is usually functional. In older lesions, BBB is almost always impaired.2
MS is characterized by infiltration of the brain by immune cells that initially invade the perivascular space surrounding the postcapillary venules. By crossing the basal lamina, the immune cells gain access to the parenchyma in large quantities by leukocytes secreting cytokines that trigger the expression of leukocyte adhesion molecules on the brain endothelial cells.2
The primary sites of immune control of the brain are the blood-CSF barriers of the choroid plexus and the meninges. In the MS rodent model of autoimmune encephalomyelitis (EAE), both are important sites of initial lymphocyte activation.2
Natalizumab prevents the interaction of the α4 integrin of immune cells with the endothelial VCAM1. This inhibits the transport of immune cells through the BBB and thus significantly reduces the formation of new lesions.39
8.4. Blood-brain barrier and epilepsy
In epilepsy, there are a number of markers for involvement of the blood-brain barrier:2
- The MRI shows a leak in the blood-brain barrier
- Parenchymal albumin is elevated in brain tissue, indicating extravasation of large molecules from the blood into the brain
- GLUT1 is regionally reduced, glucose uptake and glucose metabolism in seizure foci are reduced
Disorders of the BBB can also cause or promote epileptic seizures. Osmotic shocks, which make the blood-brain barrier permeable, have led to seizures in patients. A GLUT1 deficiency in the BBB can trigger epilepsy.2
8.5. Blood-brain barrier and Alzheimer’s disease
There are also several indications of a connection with the blood-brain barrier in Alzheimer’s disease. Profaci et al.2
8.6. Blood-brain barrier and stroke/ischemia
A stroke is associated with two phases of BBB dysfunction.2
within a few hours, an initial reduction in the permeability of the BBB occurs (first phase).
This soon decreases again, but reappears the following day (second phase).
During the first phase, transcytosis of non-specific molecules increases, followed by structural changes in the tight junctions.
A knockout of leukocyte adhesion molecules or antibodies against leukocyte adhesion molecules appear to reduce the infarct volume.2
During the second phase, most of the neurons that die during a stroke die.
Daneman R, Prat A (2015): The blood-brain barrier. Cold Spring Harb Perspect Biol. 2015 Jan 5;7(1):a020412. doi: 10.1101/cshperspect.a020412. PMID: 25561720; PMCID: PMC4292164. REVIEW ↥ ↥ ↥ ↥ ↥ ↥ ↥ ↥ ↥ ↥ ↥ ↥ ↥ ↥ ↥ ↥ ↥
Profaci CP, Munji RN, Pulido RS, Daneman R (2020): The blood-brain barrier in health and disease: Important unanswered questions. J Exp Med. 2020 Apr 6;217(4):e20190062. doi: 10.1084/jem.20190062. PMID: 32211826; PMCID: PMC7144528. REVIEW ↥ ↥ ↥ ↥ ↥ ↥ ↥ ↥ ↥ ↥ ↥ ↥ ↥ ↥ ↥
DocCheck Flexikon; Blut-Hirn-SchrankeDocCheck Flexikon; Blut-Hirn-Schranke german ↥
González-Mariscal L, Betanzos A, Nava P, Jaramillo BE (2003): Tight junction proteins. Prog Biophys Mol Biol. 2003 Jan;81(1):1-44. doi: 10.1016/s0079-6107(02)00037-8. PMID: 12475568. REVIEW ↥ ↥
Schwab, Matthias; Marx, Claudia; Zanger, Ulrich M.; Eichelbaum, Michel; Fischer-Bosch, Margarete (2002): Pharmakogenetik der Zytochrom-P-450-Enzyme: Bedeutung für Wirkungen und Nebenwirkungen von Medikamenten. Dtsch Arztebl 2002; 99(8): A-497 / B-400 / C-377 ↥
Tărlungeanu DC, Deliu E, Dotter CP, Kara M, Janiesch PC, Scalise M, Galluccio M, Tesulov M, Morelli E, Sonmez FM, Bilguvar K, Ohgaki R, Kanai Y, Johansen A, Esharif S, Ben-Omran T, Topcu M, Schlessinger A, Indiveri C, Duncan KE, Caglayan AO, Gunel M, Gleeson JG, Novarino G (2016): Impaired Amino Acid Transport at the Blood Brain Barrier Is a Cause of Autism Spectrum Disorder. Cell. 2016 Dec 1;167(6):1481-1494.e18. doi: 10.1016/j.cell.2016.11.013. PMID: 27912058; PMCID: PMC5554935. ↥ ↥ ↥ ↥
Montemitro C, Angebrandt A, Wang TY, Pettorruso M, Abulseoud OA (2021): Mechanistic insights into the efficacy of memantine in treating certain drug addictions. Prog Neuropsychopharmacol Biol Psychiatry. 2021 Dec 20;111:110409. doi: 10.1016/j.pnpbp.2021.110409. PMID: 34324921. REVIEW ↥
Medin T, Medin H, Hefte MB, Storm-Mathisen J, Bergersen LH (2019): Upregulation of the lactate transporter monocarboxylate transporter 1 at the blood-brain barrier in a rat model of attention-deficit/hyperactivity disorder suggests hyperactivity could be a form of self-treatment. Behav Brain Res. 2019 Mar 15;360:279-285. doi: 10.1016/j.bbr.2018.12.023. PMID: 30550949. ↥
Stöcker (2019): Adhäsionsmoleküle. Lexikon der Medizinischen Laboratoriumsdiagnostik. Springer. ↥
Lécuyer MA, Saint-Laurent O, Bourbonnière L, Larouche S, Larochelle C, Michel L, Charabati M, Abadier M, Zandee S, Haghayegh Jahromi N, Gowing E, Pittet C, Lyck R, Engelhardt B, Prat A (2017): Dual role of ALCAM in neuroinflammation and blood-brain barrier homeostasis. Proc Natl Acad Sci U S A. 2017 Jan 24;114(4):E524-E533. doi: 10.1073/pnas.1614336114. PMID: 28069965; PMCID: PMC5278491. ↥
Kim BJ, Hancock BM, Bermudez A, Del Cid N, Reyes E, van Sorge NM, Lauth X, Smurthwaite CA, Hilton BJ, Stotland A, Banerjee A, Buchanan J, Wolkowicz R, Traver D, Doran KS (2015): Bacterial induction of Snail1 contributes to blood-brain barrier disruption. J Clin Invest. 2015 Jun;125(6):2473-83. doi: 10.1172/JCI74159. PMID: 25961453; PMCID: PMC4497739. ↥
Prat A, Biernacki K, Wosik K, Antel JP (2001): Glial cell influence on the human blood-brain barrier. Glia. 2001 Nov;36(2):145-55. doi: 10.1002/glia.1104. PMID: 11596123. REVIEW ↥
Collignon A, Dion-Albert L, Ménard C, Coelho-Santos V (2024): Sex, hormones and cerebrovascular function: from development to disorder. Fluids Barriers CNS. 2024 Jan 4;21(1):2. doi: 10.1186/s12987-023-00496-3. PMID: 38178239; PMCID: PMC10768274. REVIEW ↥ ↥
Chi OZ, Barsoum S, Wen Y, Liu X, Weiss HR (2004): 17beta-estradiol prevents blood-brain barrier disruption induced by VEGF. Horm Metab Res. 2004 May;36(5):272-6. doi: 10.1055/s-2004-814478. PMID: 15156404. ↥
Mueller MD, Vigne JL, Minchenko A, Lebovic DI, Leitman DC, Taylor RN (2000): Regulation of vascular endothelial growth factor (VEGF) gene transcription by estrogen receptors alpha and beta. Proc Natl Acad Sci U S A. 2000 Sep 26;97(20):10972-7. doi: 10.1073/pnas.200377097. PMID: 10995484; PMCID: PMC27133. ↥
Masi A, Breen EJ, Alvares GA, Glozier N, Hickie IB, Hunt A, Hui J, Beilby J, Ravine D, Wray J, Whitehouse AJO, Guastella AJ (2017): Cytokine levels and associations with symptom severity in male and female children with autism spectrum disorder. Mol Autism. 2017 Dec 2;8:63. doi: 10.1186/s13229-017-0176-2. PMID: 29214007; PMCID: PMC5712192. ↥
Seidner G, Alvarez MG, Yeh JI, O’Driscoll KR, Klepper J, Stump TS, Wang D, Spinner NB, Birnbaum MJ, De Vivo DC (1998): GLUT-1 deficiency syndrome caused by haploinsufficiency of the blood-brain barrier hexose carrier. Nat Genet. 1998 Feb;18(2):188-91. doi: 10.1038/ng0298-188. PMID: 9462754. ↥
Klepper J, Voit T (2002): Facilitated glucose transporter protein type 1 (GLUT1) deficiency syndrome: impaired glucose transport into brain– a review. Eur J Pediatr. 2002 Jun;161(6):295-304. doi: 10.1007/s00431-002-0939-3. PMID: 12029447. REVIEW ↥
Vatine GD, Al-Ahmad A, Barriga BK, Svendsen S, Salim A, Garcia L, Garcia VJ, Ho R, Yucer N, Qian T, Lim RG, Wu J, Thompson LM, Spivia WR, Chen Z, Van Eyk J, Palecek SP, Refetoff S, Shusta EV, Svendsen CN (2017): Modeling Psychomotor Retardation using iPSCs from MCT8-Deficient Patients Indicates a Prominent Role for the Blood-Brain Barrier. Cell Stem Cell. 2017 Jun 1;20(6):831-843.e5. doi: 10.1016/j.stem.2017.04.002. PMID: 28526555; PMCID: PMC6659720. ↥
Argaw AT, Asp L, Zhang J, Navrazhina K, Pham T, Mariani JN, Mahase S, Dutta DJ, Seto J, Kramer EG, Ferrara N, Sofroniew MV, John GR (2012): Astrocyte-derived VEGF-A drives blood-brain barrier disruption in CNS inflammatory disease. J Clin Invest. 2012 Jul;122(7):2454-68. doi: 10.1172/JCI60842. PMID: 22653056; PMCID: PMC3386814. ↥
Chiaretti A, Genovese O, Aloe L, Antonelli A, Piastra M, Polidori G, Di Rocco C (2005): Interleukin 1beta and interleukin 6 relationship with paediatric head trauma severity and outcome. Childs Nerv Syst. 2005 Mar;21(3):185-93; discussion 194. doi: 10.1007/s00381-004-1032-1. PMID: 15455248. ↥
Joseph JK, Devu BK (2019): Prevalence of attention-deficit hyperactivity disorder in India: a systematic review and meta-analysis. Indian J Psychiatr Nurs. (2019) 16:118. doi: 10.4103/IOPN.IOPN_31_19, zitiert nach Kisku A, Nishad A, Agrawal S, Paliwal R, Datusalia AK, Gupta G, Singh SK, Dua K, Sulakhiya K (2024): Recent developments in intranasal drug delivery of nanomedicines for the treatment of neuropsychiatric disorders. Front Med (Lausanne). 2024 Sep 19;11:1463976. doi: 10.3389/fmed.2024.1463976. PMID: 39364023; PMCID: PMC11446881. REVIEW ↥
Ruocco LA, de Souza Silva MA, Topic B, Mattern C, Huston JP, Sadile AG (2009): Intranasal application of dopamine reduces activity and improves attention in Naples High Excitability rats that feature the mesocortical variant of ADHD. Eur Neuropsychopharmacol. 2009 Oct;19(10):693-701. doi: 10.1016/j.euroneuro.2009.02.005. PMID: 19328660. ↥
Ahirwar LK, Blackburn SL, McBride DW, T PK (2025): Reviewing vascular influences on neuronal migration, cortical development, and neurodevelopmental disorders: focus on autism, ADHD and schizophrenia. Mol Psychiatry. 2025 Dec;30(12):5953-5966. doi: 10.1038/s41380-025-03200-z. PMID: 40897863; PMCID: PMC12602333. REVIEW ↥
Fang Z, Shen G, Amin N, Lou C, Wang C, Fang M (2023): Effects of Neuroinflammation and Autophagy on the Structure of the Blood-Brain Barrier in ADHD Model. Neuroscience. 2023 Oct 15;530:17-25. doi: 10.1016/j.neuroscience.2023.08.025. PMID: 37625689. ↥
Johansson J, Landgren M, Fernell E, Vumma R, Åhlin A, Bjerkenstedt L, Venizelos N (2011): Altered tryptophan and alanine transport in fibroblasts from boys with attention-deficit/hyperactivity disorder (ADHD): an in vitro study. Behav Brain Funct. 2011 Sep 24;7:40. doi: 10.1186/1744-9081-7-40. PMID: 21942982; PMCID: PMC3191351. n = 27 ↥
Rahman MT, Ghosh C, Hossain M, Linfield D, Rezaee F, Janigro D, Marchi N, van Boxel-Dezaire AHH (2018): IFN-γ, IL-17A, or zonulin rapidly increase the permeability of the blood-brain and small intestinal epithelial barriers: Relevance for neuro-inflammatory diseases. Biochem Biophys Res Commun. 2018 Dec 9;507(1-4):274-279. doi: 10.1016/j.bbrc.2018.11.021. PMID: 30449598. ↥
Özyurt, Öztürk, Appak, Arslan, Baran, Karakoyun, Tufan, Pekcanlar (2018): Increased zonulin is associated with hyperactivity and social dysfunctions in children with attention deficit hyperactivity disorder. Compr Psychiatry. 2018 Nov;87:138-142. doi: 10.1016/j.comppsych.2018.10.006. n = 81 ↥
Çakir A, Dogru H, Laloglu E (2023): Serum Occludin and Zonulin Levels in Children With Attention-Deficit/Hyperactivity Disorder and Healthy Controls. Indian Pediatr. 2023 Feb 15;60(1):137-141. PMID: 36786182. ↥ ↥
Aydoğan Avşar P, Işık Ü, Aktepe E, Kılıç F, Doğuç DK, Büyükbayram Hİ. Serum zonulin and claudin-5 levels in children with attention-deficit/hyperactivity disorder (2021): Int J Psychiatry Clin Pract. 2021 Mar;25(1):49-55. doi: 10.1080/13651501.2020.1801754. PMID: 32757874. ↥ ↥
Lee SY, Li SC, Yang CY, Kuo HC, Chou WJ, Wang LJ (2023): Gut Leakage Markers and Cognitive Functions in Patients with Attention-Deficit/Hyperactivity Disorder. Children (Basel). 2023 Mar 5;10(3):513. doi: 10.3390/children10030513. PMID: 36980071; PMCID: PMC10047799. ↥ ↥ ↥
Asbjornsdottir B, Snorradottir H, Andresdottir E, Fasano A, Lauth B, Gudmundsson LS, Gottfredsson M, Halldorsson TI, Birgisdottir BE (2020): Zonulin-Dependent Intestinal Permeability in Children Diagnosed with Mental Disorders: A Systematic Review and Meta-Analysis. Nutrients. 2020 Jul 3;12(7):1982. doi: 10.3390/nu12071982. PMID: 32635367; PMCID: PMC7399941. METASTUDY ↥
Ling X, Linglong P, Weixia D, Hong W (2016): Protective Effects of Bifidobacterium on Intestinal Barrier Function in LPS-Induced Enterocyte Barrier Injury of Caco-2 Monolayers and in a Rat NEC Model. PLoS One. 2016 Aug 23;11(8):e0161635. doi: 10.1371/journal.pone.0161635. PMID: 27551722; PMCID: PMC4995054. ↥
Khailova L, Dvorak K, Arganbright KM, Halpern MD, Kinouchi T, Yajima M, Dvorak B (2009): Bifidobacterium bifidum improves intestinal integrity in a rat model of necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol. 2009 Nov;297(5):G940-9. doi: 10.1152/ajpgi.00141.2009. PMID: 20501441; PMCID: PMC2777452. ↥
Sayed SZ, Hassan ZO, Abdelraheem WM, Refaat RS, Abuelela IS (2024): Is there a link between peripheral inflammation and blood brain barrier integrity in children with attention-deficit/hyperactivity disorder? A case-control study. BMC Pediatr. 2024 Nov 26;24(1):769. doi: 10.1186/s12887-024-05254-4. PMID: 39592970; PMCID: PMC11590277. ↥ ↥
Maridaki Z, Syrros G, Gianna Delichatsiou S, Warsh J, Konstantinou GN (2025): Claudin-5 and occludin levels in patients with psychiatric disorders - A systematic review. Brain Behav Immun. 2025 Jan;123:865-875. doi: 10.1016/j.bbi.2024.11.006. PMID: 39500414. REVIEW ↥ ↥
Guttmann CR, Rousset M, Roch JA, Hannoun S, Durand-Dubief F, Belaroussi B, Cavallari M, Rabilloud M, Sappey-Marinier D, Vukusic S, Cotton F (2016): Multiple sclerosis lesion formation and early evolution revisited: A weekly high-resolution magnetic resonance imaging study. Mult Scler. 2016 May;22(6):761-9. doi: 10.1177/1352458515600247. PMID: 26362901. ↥
Miller DH, Khan OA, Sheremata WA, Blumhardt LD, Rice GP, Libonati MA, Willmer-Hulme AJ, Dalton CM, Miszkiel KA, O’Connor PW; International Natalizumab Multiple Sclerosis Trial Group (2003): A controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 2003 Jan 2;348(1):15-23. doi: 10.1056/NEJMoa020696. PMID: 12510038. ↥
