MPH Part 4: Preparations, Miscellaneous
- 15. Long-term effects: No habituation effects with MPH
- 16. Gender-specific differences in MPH
- 17. Areas of application of methylphenidate in relation to other ADHD medications
- 18. Methylphenidate for other disorders
- 19. Taking methylphenidate abroad
- 20. MPH medication significantly reduces the risk of addiction
- 21. Methylphenidate preparations
- 22. Drug level curves of various MPH preparations
- 23. Abuse by pupils and students during examination phases
- 24. Preparations with the same active ingredient, pharmacies and discount agreements in Germany
- 25. Methylphenidate and drug screenings
- 26. Different MPH preparations have different individual effects - despite having the same active ingredient
- 27. Further sources on methylphenidate
15. Long-term effects: No habituation effects with MPH
A study of people with ADHD who took MPH for 2 years showed a significant worsening of hyperactivity and inattention when MPH was discontinued, which corresponds to a recurrence of the symptoms improved by MPH.1
A placebo-controlled discontinuation trial in persons with ADHD who had taken MPH for over 2 years showed that discontinuation of MPH caused a significant increase in ADHD symptoms.2 Nevertheless, in some persons with ADHD it seems that after some time a continuation of the medication is dispensable, which justifies regular discontinuation attempts.
A meta-analysis of 87 randomized, placebo-controlled, double-blind studies found no evidence of a decrease in the effect of methylphenidate, amphetamine drugs, atomoxetine or α2 antagonists with prolonged use.3
A meta-analysis found only one RCT comparing the effect of a single dose of MPH with the effect of prolonged use (here: 4 weeks). This RCT found a significant agreement between the effect of a single dose and the effect of prolonged use. This is relevant for the evaluation of studies on the effect of single doses of MPH.4
16. Gender-specific differences in MPH
One study observed a higher increase in dopamine in the ventral striatum (including the nucleus accumbens) in women than in men. The increase in the dorsal striatum was the same.5
17. Areas of application of methylphenidate in relation to other ADHD medications
- According to the current European consensus, methylphenidate is the first choice of medication for ADHD in children (before amphetamine medication) and the second choice of medication in adults (after amphetamine medication)67
- In children who are MPH non-responders, i.e. who do not respond to MPH, the efficacy of amphetamine medication should be tested.
- People with ADHD with pronounced dysphoria during inactivity or with comorbid depression benefit particularly from amphetamine medication.
- In addition, people with ADHD who require stronger activation may be able to cope better with amphetamine medication.
- Highly gifted people are said to respond better to amphetamine medication than to MPH.8
18. Methylphenidate for other disorders
MPH proved to be helpful in the treatment of attention problems in children with brain injuries (traumatic brain injury).9
MPH is also used successfully for narcolepsy. In 2007, Ritalin® was the only MPH preparation approved for the treatment of narcolepsy.10
In children with ASD, MPH also appears to increase cognitive processing speed - in contrast to children with ADHD.11
19. Taking methylphenidate abroad
See in detail in the article Taking stimulants abroad
20. MPH medication significantly reduces the risk of addiction
The updated European consensus on the diagnosis and treatment of ADHD from 2018 concludes that stimulants significantly reduce the risk of addiction while taking them.12
People with ADHD with comorbid cocaine addiction showed a significant reduction in addictive behavior when treated with stimulants. This corresponded to the reduction in ADHD symptoms.13
The international consensus on screening, diagnosis and treatment of adults with addiction and ADHD recommends treatment with long-acting stimulants with increasing doses up to high doses, in combination with psychotherapeutic treatment.14
A meta-analysis of 6 studies with n = 1,014 test subjects showed a significantly reduced risk of later addiction for participants medicated with stimulants (here: MPH).15 The risk of later addiction, whether to alcohol or other substances, was found to be 1.9 times lower, i.e. almost halved.16
A Swedish cohort study found that 3 years after being prescribed stimulants for ADHD, the risk of an addiction diagnosis was reduced by 31%.17
These findings may be supported by the fact that adolescents with marked novelty-seeking who were found to have decreased BOLD activity in mesolimbic (nucleus accumbens (ventral striatum) and midbrain) and prefrontal cortical (dlPFC) regions during reward anticipation at age 14 were more likely to engage in problematic drug use at age 16.18 This could be interpreted to mean that ADHD medications that increase dopamine and norepinephrine levels, such as MPH or amphetamine medications, directly contribute to lowering the risk of addiction.
ADHD medication reduced the influence of preference for short-term rewards and frustration intolerance on internet addiction.19
One study found no increased addictive affinity in adult rodents as a result of treatment with MPH during adolescence.20 Other studies also found evidence that stimulants do not increase the risk of addiction.21
We believe that the two components of ADHD and addiction can be weighted very differently in different individuals and that the success of drug treatment depends heavily on this different weighting. The setting in which medication takes place and whether or not it is flanked and intensively supported by other measures is therefore likely to play a very important role. The practical experience of one of our advisory boards is that pure substance treatment without intensive, accompanying co-treatment usually only leads to the use of an additional substance.
In addition, a combination of addiction and ADHD often involves other comorbidities such as depression etc., which makes treatment even more difficult, as antidepressants are often no longer effective when many substances are abused. It therefore seems important to us for the success of the treatment that inpatient detoxification and drug abstinence for drug cessation are started in the case of a combination of addiction and ADHD.
“The drug treatment of patients with ADHD who also have substance abuse or dependence should be carried out by a specialist with knowledge of the treatment of ADHD and addiction.”22
Finally, it should be noted that people with ADHD often find it difficult enough to take the stimulants prescribed to them regularly and on time. If stimulants triggered addictive behavior, this would not be the case. In addition, people with ADHD do not wake up in the morning with a craving for their medication. However, such cases are not reported.
The rumors that ADHD medications would trigger addictive behavior are simply a consequence of insufficient knowledge of the differences between drug and medication. Doctors who spread such misconceptions harm their patients and risk medical malpractice claims if damage results from such advice that is contrary to the guidelines.
In our view, a neurophysiological explanation for the reduced risk of addiction due to MPH could be that MPH increases dopamine, while dopamine deficiency is associated with increased expression of the CB1 cannabinoid receptor.232425 In our opinion, however, this is contradicted by the fact that there is no known increased risk of THC addiction in Parkinson’s disease.
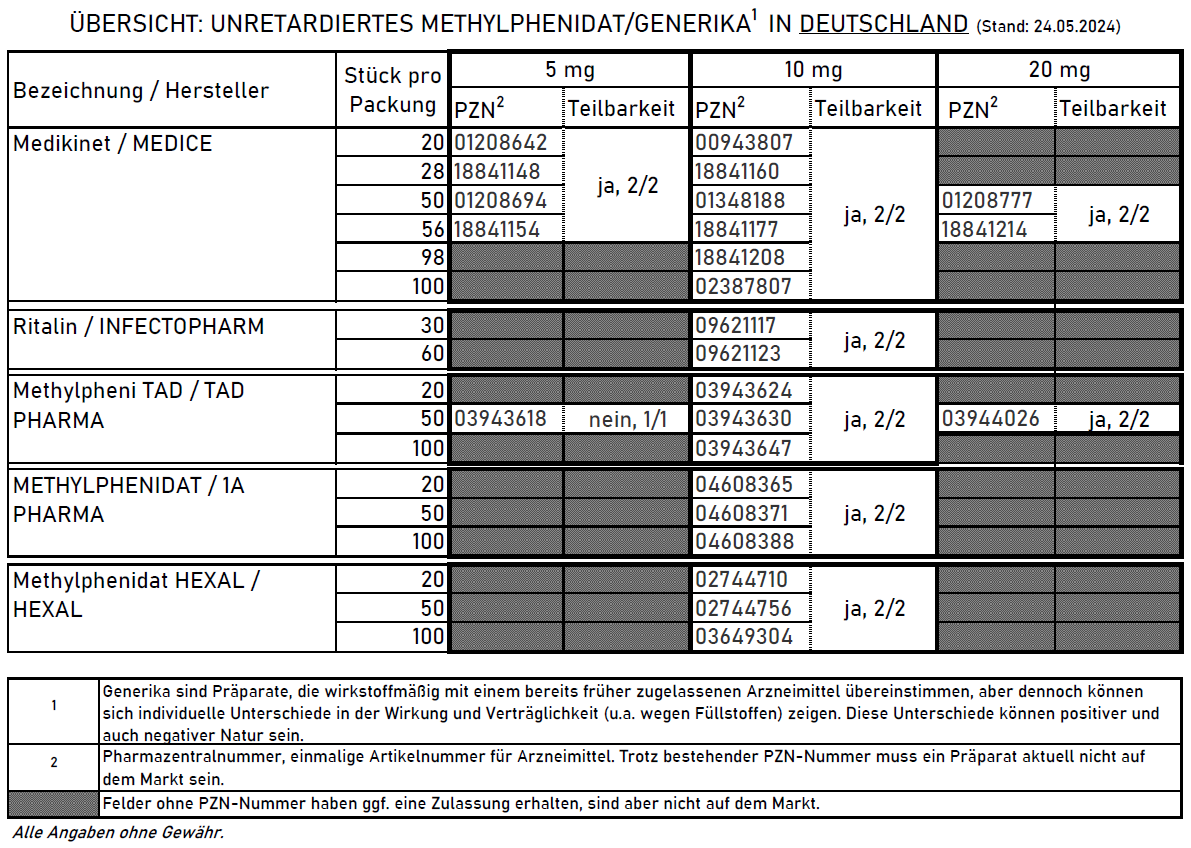
21. Methylphenidate preparations
There are a large number of methylphenidate preparations.
Although they all contain the same active ingredient, people with ADHD respond differently to them. Individual means that some people tolerate preparation A very well and it works well, while preparation B barely works and has unpleasant side effects, while for others the effect is exactly the opposite.26
A solid MPH medication regimen should therefore always include taking different preparations, even if one preparation is already effective and has no unpleasant side effects. This is because it is only possible to determine whether another preparation works significantly better after it has been tested.
Depending on the preparation, a high-fat food intake before / during ingestion can delay or accelerate the maximum effect and reduce or increase the intensity of the effect.26 We have no concrete data on this for the preparations available in Europe.
21.1. Immediate release preparations
- In general:
Preparations USA (as of 2021):
- Focalin®
- Dexmethylphenidate (hydrochloride)
- Tablet
- 3 to 5 hours
- 2.5 mg, 5 mg, 10 mg
- Methylin® Oral Solution
- Methylphenidate (hydrochloride)
- Liquid
- 3 to 5 hours
- 5 mg/5 ml, 10 mg/5 ml
- Ritalin®
- Methylphenidate (hydrochloride)
- Tablet
- 3 to 5 hours (in practice usually 2.5 to 3.5 hours)
- 5 mg, 10 mg, 20 mg
- Various generics
Immediate release preparations Germany:
- Ritalin® immediate release
- 1-3 hours working time
- Carrier substance: Wheat starch29
- Methylphenidate HEXAL®
- 1-3 hours working time
- Methylpheni TAD® immediate release
- 1-3 hours working time
- People with ADHD reported poorer tablet divisibility than with 1A, for example
- Medikinet® immediate release
- 1-3 hours working time
- Carrier substance: corn starch29
- Various generics
One (only) pharmacy in Switzerland produces MPH drops. These are even easier to dose, so that even people with ADHD who require very low doses, such as young children, can adjust the dosage precisely. Ryffel reports on one application.3031
Graphic: Brainbuzz. Thank you!
21.2. Sustained release preparations
21.2.1. Half-day dosing
21.2.1.1. Two-phase retardation / Extended release (XR) / Long acting (LA)
Preparations USA (as of 2021):
- Metadate CD® (identical to Equasym XL, EU)
- Methylphenidate (hydrochloride)
- prolonged release
- Capsule
- 8 hours
- 10 mg, 20 mg, 30 mg, 50 mg
- Metadate® ER
- Methylphenidate (hydrochloride)
- prolonged release
- Tablet
- 8 to 12 hours
- 20 mg
- Methylin® ER
- Methylphenidate (hydrochloride)
- prolonged release
- Tablet
- 8 hours
- 10 mg, 20 mg
- QuilliChew ER™
- Methylphenidate (hydrochloride)
- prolonged release
- Chewable tablet
- 8 to 12 hours
- 20 mg, 30 mg, 40 mg
- Quillivant XR®
- Methylphenidate (hydrochloride)
- prolonged release
- Liquid
- 8, 10 and 12 hours
- 25 mg / 5ml (5 mg/ml)
- Ritalin LA®
- Methylphenidate (hydrochloride)
- prolonged release
- Capsule
- 8 hours
- 10 mg, 20 mg, 30 mg, 40 mg
Half-day retarded preparations Germany:
- Equasym Retard/XL®
- Medikinet Adult®
- Medikinet Retard®
- Must be taken with food
- Bioequivalent to Medikinet Adult33
- Gluten free
- Contains lactose
- Duration until onset of action: 30 minutes27
- Duration of action 6 (to 8) hours2728
- Effect profile:28
- Maximum after 2.5 hours
- Then drop until after 4 hours
- Plateau from hour 4 to hour 6 (lower than maximum)
- After that waste to the end of operation
- Food intake required before / during ingestion27
- Capsule may be opened27
- Contraindicated when taking proton pump inhibitors at the same time34 - then use Ritalin, for example
- Ritalin LA®
- Bioequivalent to Ritalin Adult
- Duration of effect: 8 hours27 / 6-8 hours26
- Effect profile:28
- Proprietary retardation system SODAS (Spheroidal Oral Drug Absorption System)28
- No food intake required before / during ingestion27
- Delayed maximum effect when taken with / on a high-fat meal26
- Capsule may be opened and sprinkled on food2826
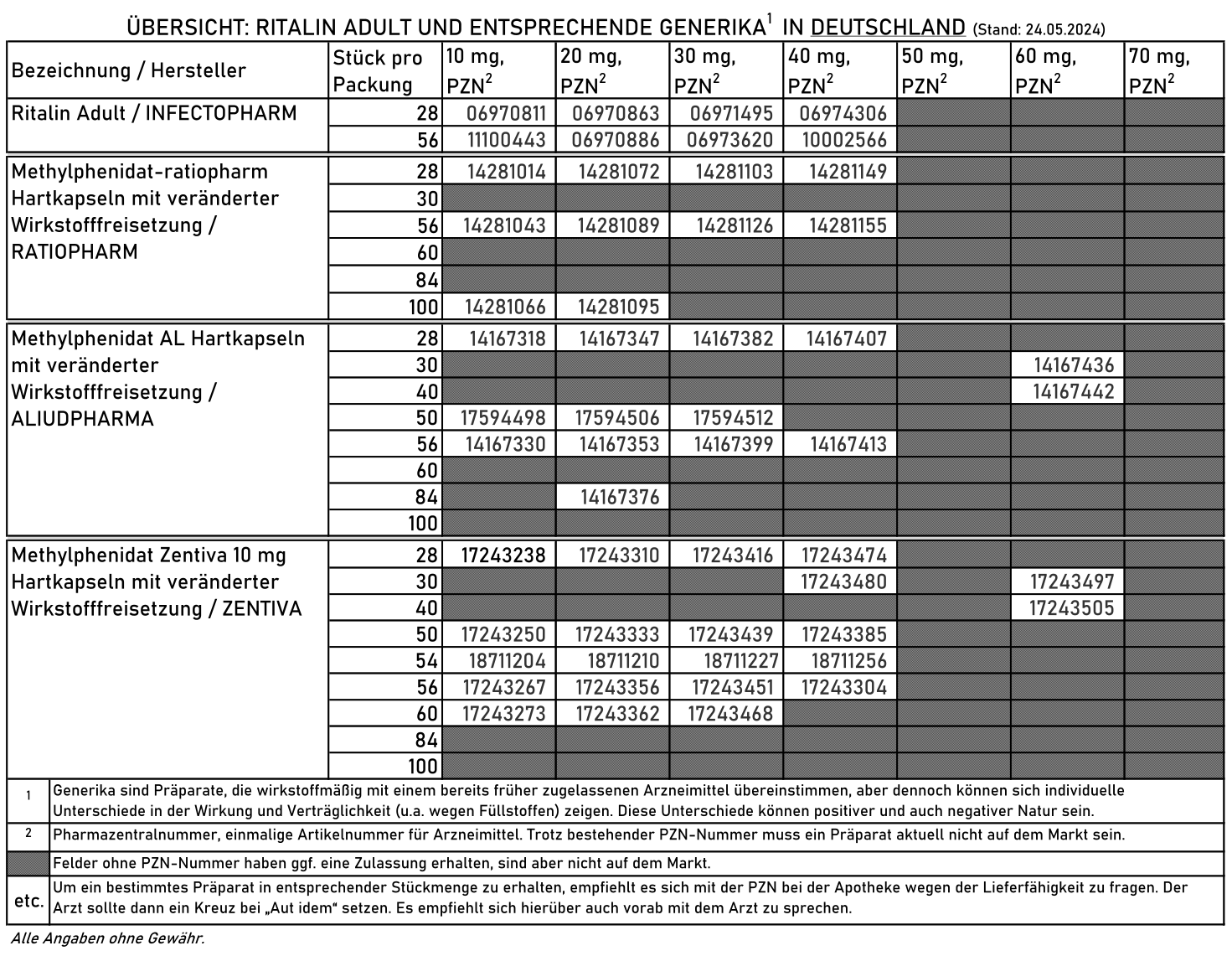
- Ritalin Adult®
- Methysym®
- Since 01.06.2021 in D
- 30 % immediate release, 70 % sustained release
- 10, 20, 30, 40, 50, 60 mg
- Up to 8 hours working time
- No food intake required for ingestion
Graphic: Brainbuzz. Thank you!
21.2.1.2. Continuous release
- Ritalin SR®
21.2.2. All-day retardation
Preparations USA:
All-day retarded preparations USA:
- Adhansia XR™
- Methylphenidate (hydrochloride)
- prolonged release
- Capsule
- 16 hours
- 25 mg, 35 mg, 45 mg, 55 mg, 70 mg, 85 mg
- Azstarys® (KP415)3536
- Mixture of
- 70 % serdex methylphenidate (SDX, prodrug of dexmethylphenidate) and
- 30 % dexmethylphenidate (d-MPH) with immediate release (IR)
- 26.1 / 5, 2mg; 39.2 / 7.8 mg; 52.3 / 10.4 mg SDX/d-MPH
- 13 hours working time
- Approval 2021
- No food intake required with ingestion, but prolongs effect
- Degradation of SDX: unknown enzymes
- Degradation of d-MPH by CES1
- No interaction with CYP2D6, CYP1A2, CYP2C8, CYP2C9, CYP2C19, CYP2E1 or CYP3A
- No interaction with alcohol
- SDX does not prolong the QT interval to a clinically relevant extent
- Mixture of
- Aptensio XR™
- Methylphenidate (hydrochloride)
- prolonged release
- Capsule
- 12 hours
- 10 mg, 15 mg, 20 mg, 30 mg, 40 mg, 50 mg, 60 mg
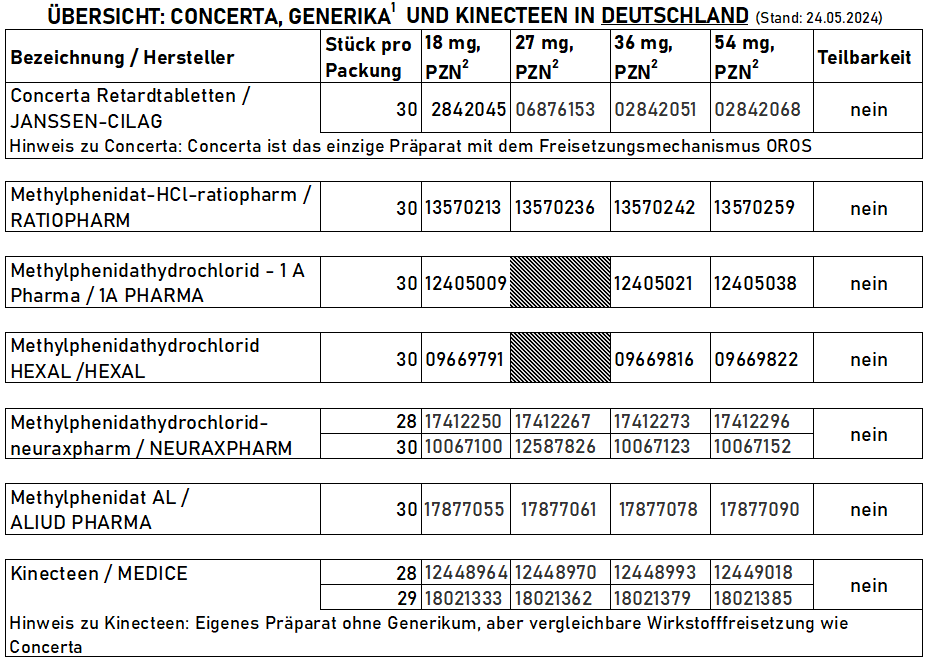
- Concerta®
- Methylphenidate (hydrochloride)
- prolonged release
- Tablet (OROS)
- 10 to 12 hours
- 18 mg, 27 mg, 36 mg, 54 mg, 72 mg
- Cotempla™XRODT
- Methylphenidate
- prolonged release
- Tablet disintegrating in the mouth
- 8 to 12 hours
- 8.6 mg, 17.3 mg, 25.9 mg
- Daytrana®
- Methylphenidate
- Transdermal patch
- Duration until onset of action approx. hours
- 10 to 16 hours
- 10 mg / 9 h (1.1 mg/h)
- 15 mg / 9 h (1.6 mg/h)
- 20 mg / 9 h (2.2 mg/h)
- 30 mg / 9 h (3.3 mg/h)
- Focalin XR®
- Dexmethylphenidate (hydrochloride)
- prolonged release
- Capsule
- 12 hours
- 5 mg, 10 mg, 15 mg, 20 mg, 25 mg, 30 mg, 35 mg, 40 mg
- Jornay PM™
- Methylphenidate (hydrochloride)
- prolonged release
- Capsule
- 12+ hours
- 20 mg, 40 mg, 60 mg, 80 mg, 100 mg
All-day retarded preparations Germany, Austria, Switzerland:
- Concerta®
- MMethylphenidate hydrochloride
- Extended-release tablets
- 18, 27, 36, 54 mg
- Due to the design, a residue of the active ingredient remains in the capsule and is not absorbed
- Therefore 18 mg corresponds to an intake of approx. 15 mg (27 mg = approx. 22.5 mg, 36 mg = approx. 30 mg, 54 mg = approx. 45 mg)
- Bioequivalent: methylphenidate hydrochloride-neuraxpharm
- Duration until onset of action: 60 minutes27
- Therefore often good effect with simultaneous intake of small dose of immediate release MPH
- Ingestion with / after food can delay maximum effect (Tmax) by up to 1 hour and reduce it by 10 to 30 % (serum concentration maximum, Cmax)26
- Duration of action 8 to 12 hours,27 10 to 12 hours2628
- Effect profile:
- Intake:
- Methylphenidate hydrochloride-neuraxpharm
- 12 hours working time
- Bioequivalent to Concerta
- No food intake required before / during ingestion
- Kinecteen® (D/A/CH), Rubicrono® (Spain), Xaggitin® (United Kingdom)
- 12 hours working time (according to manufacturer)
- No food intake required before / during ingestion
- Must not be divided, chewed or crushed (would cancel out retardation)
- Methylphenidate hydrochloride Ratiopharm37
- 12 hours working time
- Methylphenidate hydrochloride Hexal38
- 12 hours working time
Graphic: Brainbuzz. Thank you!
The course of the effect curve differs considerably depending on the MPH preparation.
22. Drug level curves of various MPH preparations
The temporal progression of active ingredient levels of different MPH preparations can be depicted and compared in active ingredient curves.3940
A graph illustrates the course of the active ingredient levels of:41
- Concerta
- Ritalin Adult / LA
- Medikinet adult
- MPH immediate release
A graph illustrates the different active ingredient levels of Equasym with and without food intake.42
The Ratiopharm prescribing information compares the drug level profile of methylphenidate Ratiopharm 40 mg with Ritalin LA.43
This shows that even bioequivalent preparations do not have completely identical effect level curves.
The information for healthcare professionals from Novartis compares the drug level profile of Ritalin LA 40 mg with Ritalin immediate release 2 x / day.44
23. Abuse by pupils and students during examination phases
See under Abuse of prescribed stimulants In the article Stimulants (MPH, AMP) for ADHD
24. Preparations with the same active ingredient, pharmacies and discount agreements in Germany
The individual preparations often differ in terms of bioavailability, effect, duration of action and side effects, even at the same dose.
It often takes a long time to find the right individual preparation and the right dosage for the patient. If a preparation is replaced due to a discount agreement, this can have significant consequences for the therapy, including discontinuation of therapy.
Against this background, the inclusion of stimulants on the substitution exclusion list is urgently required.
25. Methylphenidate and drug screenings
Methylphenidate-containing drugs can lead to a false positive laboratory value for amphetamines, especially in immunoassay methods.45
26. Different MPH preparations have different individual effects - despite having the same active ingredient
The fact that methylphenidate preparations can have very different effects on different individuals has not yet been scientifically substantiated, but has been recognized beyond doubt empirically.46 We suspect a connection with the different active (time) profiles and various fillers.
We know people with ADHD who reacted with aggression to one MPH preparation and responded excellently to another preparation - and other people with ADHD who reacted to the two preparations with exactly the opposite reaction.
The phenomenon is widely known in forums for people with ADHD and the testing of various MPH preparations is regularly recommended by people with ADHD.
However, since the approval of Vyvanse Adult in May 2019, methylphenidate is increasingly being replaced by Vyvanse in adults. In adults, amphetamine medication is the first choice of medication over methylphenidate.
27. Further sources on methylphenidate
A detailed graphic and explanatory presentation of the pathway of action of methylphenidate can be found at www.pharmgkb.org.47 Another comprehensive description of methylphenidate can be found at drugbank.com.48
Matthijssen, Dietrich, Bierens, Kleine Deters, van de Loo-Neus, van den Hoofdakker, Buitelaar, Hoekstra (2019): Effects of Discontinuing Methylphenidate on Strengths and Difficulties, Quality of Life and Parenting Stress. J Child Adolesc Psychopharmacol. 2019 Dec 24. doi: 10.1089/cap.2019.0147. ↥
Matthijssen, Dietrich, Bierens, Kleine Deters, van de Loo-Neus, van den Hoofdakker, Buitelaar, Hoekstra (2019): Continued Benefits of Methylphenidate in ADHD After 2 Years in Clinical Practice: A Randomized Placebo-Controlled Discontinuation Study. Am J Psychiatry. 2019 Sep 1;176(9):754-762. doi: 10.1176/appi.ajp.2019.18111296. ↥
Castells, Ramon, Cunill, Olivé, Serrano (2020): Relationship Between Treatment Duration and Efficacy of Pharmacological Treatment for ADHD: A Meta-Analysis and Meta-Regression of 87 Randomized Controlled Clinical Trials. J Atten Disord. 2020 Feb 20:1087054720903372. doi: 10.1177/1087054720903372. PMID: 32075485. ↥
Parlatini V, Bellato A, Roy S, Murphy D, Cortese S (2024): Association Between Single-Dose and Longer Term Clinical Response to Stimulants in Attention-Deficit/Hyperactivity Disorder: A Systematic Review of Randomized Controlled Trials. J Child Adolesc Psychopharmacol. 2024 Jul 19. doi: 10.1089/cap.2024.0038. PMID: 39027968. ↥
Manza, Shokri-Kojori, Wiers, Kroll, Feldman, McPherson, Biesecker, Dennis, Johnson, Kelleher, Qu, Tomasi, Wang, Volkow (2021): Sex differences in methylphenidate-induced dopamine increases in ventral striatum. Mol Psychiatry. 2021 Oct 27. doi: 10.1038/s41380-021-01294-9. Epub ahead of print. PMID: 34707237. n = 95 ↥
Kooij, Bijlenga, Salerno, Jaeschke, Bitter, Balázs, Thome, Dom, Kasper, Filipe, Stes, Mohr, Leppämäki, Brugué, Bobes, Mccarthy, Richarte, Philipsen, Pehlivanidis, Niemela, Styr, Semerci, Bolea-Alamanac, Edvinsson, Baeyens, Wynchank, Sobanski, Philipsen, McNicholas, Caci, Mihailescu, Manor, Dobrescu, Krause, Fayyad, Ramos-Quiroga, Foeken, Rad, Adamou, Ohlmeier, Fitzgerald, Gill, Lensing, Mukaddes, Brudkiewicz, Gustafsson, Tania, Oswald, Carpentier, De Rossi, Delorme, Simoska, Pallanti, Young, Bejerot, Lehtonen, Kustow, Müller-Sedgwick, Hirvikoski, Pironti, Ginsberg, Félegeházy, Garcia-Portilla, Asherson (2018): Updated European Consensus Statement on diagnosis and treatment of adult ADHD, European Psychiatrie, European Psychiatry 56 (2019) 14–34, http://dx.doi.org/10.1016/j.eurpsy.2018.11.001, Seite 22, 7.4.1. ↥
Cortese, Adamo, Del Giovane, Mohr-Jensen, Hayes, Carucci, Atkinson, Tessari, Banaschewski, Coghill, Hollis, Simonoff, Zuddas, Barbui, Purgato, Steinhausen, Shokraneh, Xia, Cipriani (2018): Comparative efficacy and tolerability of medications for attention-deficit hyperactivity disorder in children, adolescents, and adults: a systematic review and network meta-analysis. Lancet Psychiatry. 2018 Sep;5(9):727-738. doi: 10.1016/S2215-0366(18)30269-4. REVIEW ↥
Castello et al. 1992, zitiert nach Arnold: Journal of Attention Disorders Vol. 3(4):200-211 (2000) Methylphenidate vs. amphetamine: Comparative review. REVIEW ↥
LeBlond, Smith-Paine, Riemersma, Horn, Wade, Kurowski (2019): Influence of Methylphenidate on Long-Term Neuropsychological and Everyday Executive Functioning After Traumatic Brain Injury in Children with Secondary Attention Problems. J Int Neuropsychol Soc. 2019 Jun 10:1-10. doi: 10.1017/S1355617719000444. ↥
Handwerker (2007): Narkolepsie. Monatsschrift Kinderheilkunde. July 2007, Volume 155, Issue 7, pp 624–629 ↥
Peled, Cassuto, Berger (2019): Processing speed as a marker to stimulant effect in clinical sample of children with high functioning autism spectrum disorder. Nord J Psychiatry. 2019 Nov 5:1-5. doi: 10.1080/08039488.2019.1686063. ↥
Kooij, Bijlenga, Salerno, Jaeschke, Bitter, Balázs, Thome, Dom, Kasper, Filipe, Stes, Mohr, Leppämäki, Brugué, Bobes, Mccarthy, Richarte, Philipsen, Pehlivanidis, Niemela, Styr, Semerci, Bolea-Alamanac, Edvinsson, Baeyens, Wynchank, Sobanski, Philipsen, McNicholas, Caci, Mihailescu, Manor, Dobrescu, Krause, Fayyad, Ramos-Quiroga, Foeken, Rad, Adamou, Ohlmeier, Fitzgerald, Gill, Lensing, Mukaddes, Brudkiewicz, Gustafsson, Tania, Oswald, Carpentier, De Rossi, Delorme, Simoska, Pallanti, Young, Bejerot, Lehtonen, Kustow, Müller-Sedgwick, Hirvikoski, Pironti, Ginsberg, Félegeházy, Garcia-Portilla, Asherson (2018): Updated European Consensus Statement on diagnosis and treatment of adult ADHD, European Psychiatrie, European Psychiatry 56 (2019) 14–34, http://dx.doi.org/10.1016/j.eurpsy.2018.11.001, Seite 22, 7.4.4. ↥
Manni, Cipollone, Pallucchini, Maremmani, Perugi, Maremmani (2019): Remarkable Reduction of Cocaine Use in Dual Disorder (Adult Attention Deficit Hyperactive Disorder/Cocaine Use Disorder) Patients Treated with Medications for ADHD. Int J Environ Res Public Health. 2019 Oct 15;16(20). pii: E3911. doi: 10.3390/ijerph16203911. ↥
Crunelle, van den Brink, Schellekens, van de Glind, Icasa Consortium, Matthys (2019): [International consensus for the screening, diagnosis and treatment of adult patients with substance use disorder and ADHD]. [Article in Dutch] Tijdschr Psychiatr. 2019;61(7):477-487. ↥
Wilens, Faraone, Biederman, Gunawardene (2003): Does stimulant therapy of attention-deficit/hyperactivity disorder beget later substance abuse? A meta-analytic review of the literature; Pediatrics. 2003 Jan;111(1):179-85. REVIEW ↥
Edel, Vollmoeller (2006): Aufmerksamkeitsdefizit-/Hyperaktivitätsstörung bei Erwachsenen, Springer, Seite 120 ↥
Chang Z, Lichtenstein P, Halldner L, D’Onofrio B, Serlachius E, Fazel S, Långström N, Larsson H (2014): Stimulant ADHD medication and risk for substance abuse. J Child Psychol Psychiatry. 2014 Aug;55(8):878-85. doi: 10.1111/jcpp.12164. PMID: 25158998; PMCID: PMC4147667. ↥
Büchel, Peters, Banaschewski, Bokde, Bromberg, Conrod, Flor, Papadopoulos, Garavan, Gowland, Heinz, Walter, Ittermann, Mann, Martinot, Paillère-Martinot, Nees, Paus, Pausova, Poustka, Rietschel, Robbins, Smolka, Gallinat, Schumann, Knutson, the IMAGEN consortium (2012): Blunted ventral striatal responses to anticipated rewards foreshadow problematic drug use in novelty-seeking adolescents; Nat Commun. 2017; 8: 14140. doi: 10.1038/ncomms14140; PMCID: PMC5321762; n = 144 ↥
Lu, Chou, Hsiao, Hu, Yen (2019): Correlations of Internet Addiction Severity With Reinforcement Sensitivity and Frustration Intolerance in Adolescents With Attention-Deficit/Hyperactivity Disorder: The Moderating Effect of Medications. Front Psychiatry. 2019 Apr 26;10:268. doi: 10.3389/fpsyt.2019.00268. eCollection 2019. ↥
Zhang-James, Lloyd, James, Yang, Richards, Faraone (2019); Oral Methylphenidate Treatment of an Adolescent ADHD Rat Model Does Not Alter Cocaine-Conditioned Place Preference during Adulthood: A Negative Report. J Psychiatr Brain Sci. 2019;4. pii: e190021. doi: 10.20900/jpbs.20190021. ↥
Ide, Ikekubo, Hua, Takamatsu, Uhl, Sora, Ikeda (2018): Reward-enhancing effect of methylphenidate is abolished in dopamine transporter knockout mice: A model of attention-deficit/hyperactivity disorder. Neuropsychopharmacol Rep. 2018 Sep;38(3):149-153. doi: 10.1002/npr2.12020. ↥
Rösler, Retz (2020): Medikamentöse Therapie der ADHS bei Erwachsenen; Psychiatrie up2date 2020; 14: 59–75 ↥
Lastres-Becker I, Cebeira M, de Ceballos ML, Zeng BY, Jenner P, Ramos JA, Fernández-Ruiz JJ (2001): Increased cannabinoid CB1 receptor binding and activation of GTP-binding proteins in the basal ganglia of patients with Parkinson’s syndrome and of MPTP-treated marmosets. Eur J Neurosci. 2001 Dec;14(11):1827-32. doi: 10.1046/j.0953-816x.2001.01812.x. PMID: 11860478. ↥
Mailleux P, Vanderhaeghen JJ (1993): Dopaminergic regulation of cannabinoid receptor mRNA levels in the rat caudate-putamen: an in situ hybridization study. J Neurochem. 1993 Nov;61(5):1705-12. doi: 10.1111/j.1471-4159.1993.tb09807.x. PMID: 7901331. ↥
Romero J, Berrendero F, Pérez-Rosado A, Manzanares J, Rojo A, Fernández-Ruiz JJ, de Yebenes JG, Ramos JA (2000): Unilateral 6-hydroxydopamine lesions of nigrostriatal dopaminergic neurons increased CB1 receptor mRNA levels in the caudate-putamen. Life Sci. 2000;66(6):485-94. doi: 10.1016/s0024-3205(99)00618-9. PMID: 10794065. ↥
Elbe, Black, McGrane, Procyshyn (Hrsg.) (2019): Clinical Handbook of Psychotrophic Drugs for Children and Adolescents, 4th edition ↥ ↥ ↥ ↥ ↥ ↥ ↥ ↥ ↥ ↥ ↥ ↥ ↥ ↥ ↥ ↥ ↥ ↥ ↥ ↥
https://www.kinderaerzte-im-netz.de/media/53ec94e833af614b730097d1/source/20080530092715_adhs2.pdf ↥ ↥ ↥ ↥ ↥ ↥ ↥ ↥ ↥ ↥ ↥ ↥ ↥ ↥ ↥ ↥ ↥
Banaschewski T, Coghill D, Santosh P, Zuddas A, Asherson P, Buitelaar J, Danckaerts M, Döpfner M, Faraone SV, Rothenberger A, Sergeant J, Steinhausen HC, Sonuga-Barke EJ, Taylor E (2006): Long-acting medications for the hyperkinetic disorders. A systematic review and European treatment guideline. Eur Child Adolesc Psychiatry. 2006 Dec;15(8):476-95. doi: 10.1007/s00787-006-0549-0. PMID: 16680409. REVIEW ↥ ↥ ↥ ↥ ↥ ↥ ↥ ↥ ↥ ↥ ↥ ↥ ↥
http://www.ads-hyperaktivitaet.de/FAQ/Infos/Medis/medis.html#1 ↥ ↥ ↥
Ryffel (2008): Vortrag beim Rheinfelder Herbstsymposium 6. November 2008 ↥
Ryffel (2003): Langzeiterfahrungen mit Stimulanzien bei ADHS: Empfehlungen für die Praxis. Forum der Kinder- und Jugendpsychiatrie und Psychotherapie, 13. J. Heft 1, S. 27 - 47, 2003 ↥
Goltz, Schröder (2021): Interaktionen mit PPI sind selten – aber es gibt Ausnahmen; Pharmazeutische Zeitung ↥ ↥
Childress AC, Marraffino A, Cutler AJ, Oh C, Brams MN (2023): Safety and Tolerability of Serdexmethylphenidate/Dexmethylphenidate Capsules in Children with Attention-Deficit/Hyperactivity Disorder: A 12-Month, Open-Label Safety Study. J Child Adolesc Psychopharmacol. 2023 Feb 20. doi: 10.1089/cap.2022.0076. PMID: 36809150. ↥
Medikamenten-Fachinformation des Herstellers ratiopharm ↥
Fachinformation Gelbe Liste Methylphenidathydrochlorid Hexal ↥
https://www.adhspedia.de/wiki/Datei:Methylphenidat-Spiegelverlauf.jpg ↥
https://www.uniklinik-ulm.de/fileadmin/default/Kliniken/Kinder-Jugendpsychiatrie/Praesentationen/Haege_2018_09_13_Curriculum_Potsdam.pdf, Seiten 17 und 18 ↥
Lenhart (2019): ADHS – Bei Erwachsenen häufig unterschätzt. Pharmazeutische Zeitung ↥
https://www.adhspedia.de/images/e/e7/Equasym-Kurve-Nahrung.jpg ↥
https://www.ratiopharm.de/assets/products/de/label/Methylphenidat-ratiopharm%20Hartkapseln%20mit%20veranderter%20Wirkstofffreisetzung%20-%2012.pdf?pzn=14281149, Seite 20 ↥
Novartis: Fachinformation Ritalin LA, unter 12.3. ↥
Gelbe Liste: Methylphenidat. Deutsch ↥
Schonwald, Chan, Nyp (2019): Not Really “The Same Thing”. J Dev Behav Pediatr. 2019 Dec 3. doi: 10.1097/DBP.0000000000000756. ↥
pharmgkb.org: Methylphenidate Pathway, Pharmacodynamics. Abruf 26.03.22 ↥
Drugbank: Methylphenidate. Abgerufen 30.03.2022 ↥
